Atlantic bluefin tuna is one of the high value aquaculture species. From the middle of the 1990s, aquaculture of Atlantic bluefin tuna was developed primarily around so-called “fattening” whereby a school of tuna are caught by purse seine fishing vessels, transferred into transport cages and towed from the capture areas to the fattening facilities. These facilities are generally standard floating cages constructed of high density polyethylene tubes (HDPE) with nylon netting. During the fattening period the fish are heavily fed with oily pelagic fishes. The farming is motivated by a highly specialized and categorized Japanese tuna market, where bluefin tuna have the highest commercial value if the meat contains desirable fat contents. The Atlantic bluefin tuna fattening in Europe started in Spain in 1996 in Murcia Region and in Croatia followed by Malta (2000), Italy (2001), Turkey (2002) and Tunisia (2003). Cyprus and Libya also ventured into bluefin tuna farming later but the operation was discontinued owing to the small quota for fishing of “seeds” and political unrest, respectively. In the Mediterranean, from 1996 to 2001, there was at least a 20-fold increase in the estimated number of cages, though this expansion may have been even greater. The export of fattened bluefin tuna to Japan started in 1997.
Bluefin tuna are large pelagic marine fish. The juveniles are encountered in epipelagic waters whereas large tunas tend to be mesopelagic and are found also in deeper and cooler waters. The species has considerable thermal tolerances, as it can be found in waters as cold as 10 °C, as well as in tropical areas. Generally the most critical environmental parameters for these large pelagic fish are sea surface temperature and the levels of dissolved oxygen and salinity. The species has been observed both above and below the thermocline. Juvenile fish tend to live near the surface. The following three growth stages can be distinguished: (i) larvae – recently hatched individuals which are considerably different in appearance from juveniles or adults; (ii) juveniles – similar in appearance to adults, but sexually immature; and (iii) adults – sexually mature fish. The maximum reported weight of an adult specimen was 684 kilograms, with a total length of 458 cm. The species seems to have an average lifespan of around 15 years, while the longevity for both the Atlantic and the southern bluefin tunas was estimated at around 20 years. For adults natural mortality rates range from 0.2 to 0.6, while natural rates for juveniles are higher. All bluefin tuna species move constantly in search for food and to maintain a constant water flow over their gills. The Atlantic bluefin tuna migrate seasonally over long distances between temperate waters, where they feed, and tropical waters, where they spawn. Spawning is generally limited to relatively restricted areas in temperate and tropical waters. Thunnus thynnus may form giant schools spreading over several nautical miles when migrating into the Mediterranean Sea to spawn during the summer months. Most bluefin school according to their size, however it is not unusual for different size groups to school together. Juveniles are, therefore, often associated with smaller tuna species such as the skipjack or bonito. While schooling is believed to be sight-oriented, schools have been observed at night. Bluefin tuna are excellent swimmers and can swim at high speed for long periods as they are able to absorb and utilize large amounts of oxygen. Their bodies are designed for high performance at both sustainable and burst swimming speeds. Tuna must swim constantly to satisfy their oxygen requirements in order to stay alive. Their swimming pattern seems to be influenced by both the distribution of food and the need to return to their ancestral spawning grounds at the appropriate time. To efficiently transfer oxygen from the gills to the other body tissues, tunas have hearts that are approximately 10 times the size of those of other fish, relative to the body weight, and blood pressure and pumping rate about three times higher. Tunas have two types of muscle, white and red. The white muscles function during short bursts of activity, while the red muscles, which have a relatively large mass, allow the fish to swim at high speeds for long periods without fatigue, as demonstrated by tagging studies with conventional and sonic tags. Tuna larvae live in warm surface waters and feed primarily on zooplankton, including small crustaceans and the larvae of crustaceans, fishes, molluscs and jellyfish. Tuna larvae are preyed upon by zooplankton foragers, such as larger larvae and early juveniles of other pelagic fish. Juvenile and adult tuna generally prey on fish, squid and crustaceans. The larger specimens, which feed on pelagic fishes, are positioned at the top of the trophic web and locate their prey visually. To satisfy their nutritional requirements tunas have to swim long distances. Their type of locomotion is particularly well adapted to the search for prey in large water volumes with the least expenditure of energy. Tuna break up schools of prey, producing disorientation and straggling. When prey is detected, the tuna change their behaviour and have a general increase of activity, e.g. increase in swimming speed, change in swimming pattern and energetic pursuit to obtain smaller schooling fish such as anchovies. The spawning of bluefin tuna has been so far detected in only two areas: the Mediterranean and the Gulf of Mexico. In the Gulf of Mexico, spawning occurs from April to June when the water temperature is 25–30 °C and in the Mediterranean from May/June to August. Spawning was also reported in the Levantine Sea (Eastern Mediterranean basin) with a peak in the activity in May. Sexual maturity of the Atlantic bluefin tuna is reached at the age of 5–8 years, while in the eastern Atlantic maturity is reached earlier, at 4–5 years. Scientists have found that in the Balearic Islands (Mediterranean) bluefin tuna are able to spawn from 3 years old. bluefin tunas may release up to 45 million eggs and spawning occurs in open water close to the surface and in areas where the survival expectations of the larvae is highest. Atlantic bluefin tuna are considered a suitable candidate species for marine aquaculture because of a strong market awareness and demand, and also because of its fast growth rate, convenient ratio of edible meat to body weight, and tolerance to a wide temperature range. However, the feeding operations for fattening require a large amount of feed.
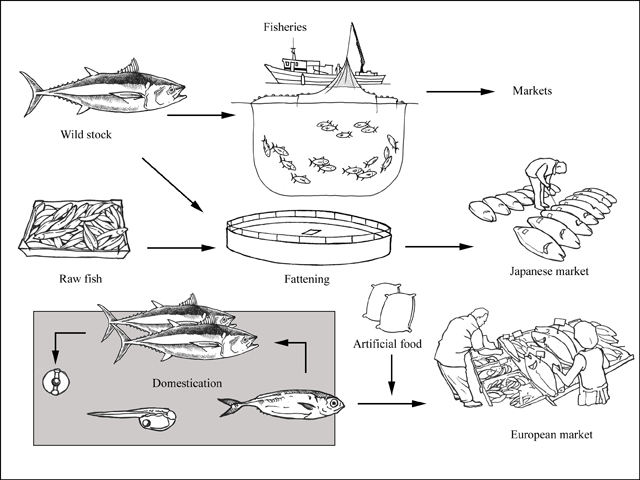
To date, all commercial tuna aquaculture rely on the wild capture of young fish for use as seeds, though artificially produced seeds starting from fertilized eggs have been successfully raised to marketable size in research institutions. Aquaculture practices using wild seeds are sometimes classified as “capture based aquaculture”. The prevailing practices in Atlantic bluefin tuna farming is more often described as fattening because the wild seeds are at least several kilograms in body weight and some are close to adult size already. The desirable result of increased fat content in the tuna meat makes the term “fattening” more relevant.
With this method, the tuna schools are caught by purse seiners in the reproductive areas in the Mediterranean. The purse seines is a unique fishing gear that allows the fisher to capture large tuna alive. Once captured, tuna are transferred into the transport cages (circular of 50 m diameter or hexagonal with 22 m sides) and towed from the capture areas to the fattening facilities. In order to avoid the collapse of the transport cages and protect the fish within them, the cages are towed at slow speed, commonly 1–1.5 knots. This avoids excessive mortality and allow tuna to swim easily. The tuna are placed in their definitive fattening units, usually in sea cages of 50–120 m diameter and 25–35 m depth. Some farms also use round-corner rectangular cages. The density of tunas inside the cage can reach 5 kg/m3, but is usually kept lower. During a period of time (usually 6 months, from July to December) the fish are fed with previously frozen feed including mainly mackerels, pilchards and some cephalopods. In general, the fishes are fed to satiation by means of a feeding hose which transfers water and feed from the boat into the centre of the cage. Alternatively, a block of frozen feed can be placed in the centre of the cage such that the pieces break off and sink as they defrost. The body weight gain during the fattening process depends of many factors including: initial size, water temperature, number of days that the tuna are fed, quality of feed (amount of fat), and amount of feed. In general, a 40–50 kg bluefin tuna could increase its weight by 30–40 percent in 6–7 months, while a bluefin tuna of 150–200 kg could increase by 12–15 percent in the same period. The variability in weight gain is similarly reflected in the variability of feed conversion ratio (FCR). Individuals with large weight at stocking, exhibit higher FCR over 40 (40 kg of feed per kg of tuna weight gain).This high FCR is because of tuna physiology and growth characteristics; indeed large tunas do not increase mass as much as they increase the fat content. Nevertheless, in small individuals (< 30 kg) some authors reported FCR of 15–20. bluefin tuna growth can be monitored and estimated by using underwater stereo-video cameras, thereby avoiding the stress and possible trauma caused by capture and handling. The fattening process commonly used in Croatia is slightly different because the tuna are captured at a smaller size and the fattening period is longer. Here, tuna are captured at 2 years old and 10 kg, and are fattened for 18–30 months, reaching 45–90 kg, respectively.
The market price of bluefin tuna is strongly correlated to the quality of the meat; slaughter, processing, storage and shipping need to be of the highest standards. Overall, the fish needs to be slaughtered, bled and cooled as quickly as possible. Every effort should be taken to prevent the formation and build-up of lactic acid and other metabolites in the muscle, which imparts a metallic taste to the flesh when eaten raw. This defect, known as “burnt flesh syndrome” or yake in Japanese, causes a dramatic decrease in sale price, and possibly complete rejection by the buyers. Lactic acid is a normal by product of muscle activity.
The technique of fattening bluefin tuna required the industry to develop new slaughtering methods to ensure that death occurs as swiftly as possible. There are two main slaughtering methods:
The sale price depends heavily on the quality of the meat. Fresh is worth more than frozen. Important factors include the amount of fat, the colour and the texture of the meat, and above all, the degree of yake.
In contrast to the fattening operations that rely on wild caught young tuna as “seeds”, full cycle aquaculture of tuna covers spawning in captivity, egg collection, incubation, larval rearing and grow out to marketable size. In Japan, only a few companies and research universities have the capacity to do so for the Pacific bluefin tuna (Thunnus orientalis). The first was Kindai (formerly Kinki) University. In 2002, it closed the life cycle of this species in captivity. In Europe, the Spanish Institute of Oceanography (IEO) in the last 20 years, is working on the research projects focus on developing suitable techniques to obtain fertilized eggs and producing Atlantic bluefin tuna juveniles in its facilities in Mazarrón (southeast Spain).
In full cycle aquaculture as practiced by IEO, eggs are obtained from captive broodstock kept in 25 m diameter and 15 m deep floating cages. The larval rearing is carried out in tanks (40 m3) over the course of about 40 days. The larval system is the so-called pseudogreen water (PGW) where cultured phytoplankton is added to the larval rearing tank each day during the critical segment of the growing process. The larvae are fed sequentially on enriched rotifers, enriched Artemia, sac larvae of gilthead seabream (Sparus aurata) and finally weaned onto artificial food or minced raw fish. Recent use of copepod nauplii (Acartia) in combination with enriched rotifers has been implemented with better results in terms of growth and survival of bluefin tuna larvae. Since 2011, the IEO has produced a few thousands of 40 days old bluefin tuna juveniles (around 5 g each) and transferred them to the grow out cages. The first batch was sent to market in November 2014 at about 20 kg each after 3–4 years. In July 2016, the IEO closed for the first time the life cycle of the bluefin tuna in captivity.
Industry wide, the mortality of the tuna fattened in the cages is generally low (less than 5 percent) and it has continued to decrease with improved management practices and technological advances. Some of these improved practices include transferring between the transport and the rearing cages, feed quality, cage size and design, and better site selection. Conversely, full cycle aquaculture of bluefin tuna has a survival rate from egg to juvenile (40 days past hatching) of less than 1 percent.
Little is known about the pathology of this species in captivity. The adult bluefin tuna seem to be quite resistant to diseases even when they are under stress, trauma and another factors. Even so, several pathologies have been described mainly due to parasites. The main important pathological events have been described in other species as southern bluefin tuna (Thunnus maccoyii). Sometimes important mortalities have been associated with severe storms with strong winds. In these cases, the tuna often displayed a large amount of mucus in their gills. Other mortality events have been reported because of toxic microalgae, turbidity, hypoxia or a combination of several of these factors. Other than that, there have been problems related to the feed. Sometimes, frozen low value feed fish have oxidized lipids that can lead to nutritional problems. In addition, improper handling of the feed can lead to contamination and subsequent infection by pathogens, both viral and bacterial.
One way to calculate the aquaculture production is to look at the bluefin tuna catch by purse seine vessels. Considering that young tunas for fattening purposes are only caught by purse seine, and that almost all fish caught by this gear are destined for fattening, the catch by purse seine could be used as a proxy for the initial stocking. Then, an average of 15 percent of weight gain on top of initial stocking weight could be applied to calculate the final production at harvest. This methodology, however, is based on several assumptions and must be treated an approximation. Being the only source of comprehensive global capture fisheries and aquaculture production statistics, FAO also collects and disseminates farmed bluefin tuna production data annually. Farmed Atlantic bluefin tuna productions in FAO aquaculture statistics are basically the results of estimates based on the data and information from a variety of sources, including producing countries report, regional fisheries management organizations (RFMO) and import data from Japan. For years the statistics unit of FAO’s Fisheries and Aquaculture Department has been well aware of reliability of Atlantic bluefin tuna aquaculture statistics and questions by internal and external data users. FAO data on farmed bluefin tuna production, including those for back years, are subject to revision upon additional or new data becoming available to FAO. It is predicted that in the next years the production of bluefin tuna through the fattening process will increase. bluefin tuna wild populations appear to be responding favourably to the implemented control and recovery program by the International Commission for the Conservation of Atlantic Tunas (ICCAT). It is expected that the quota will be increased and therefore capture will also increase. Nevertheless, owing to the level of uncertainty in stock assessment and a precautionary management by the ICCAT, there is unlikely to be a significant rise in production from capture-based aquaculture. However, some companies practicing full cycle aquaculture are poised for commercialization, though their contributions to the global production statistics are unlikely to be significant in the near future.
Japan has always been the primary market destination of cultured bluefin tuna, and nearly all bluefin tuna are sold to Japanese market. The final bluefin tuna products (mainly as sushi and sashimi) continue to show a positive trend in consumption, with prices depending on the quality of the individual fish specimen. Nevertheless, important markets in Southeast Asia, Europe and the United States of America are emerging, with competitive prices. The sale price depends of many factors, including: if it is fresh or frozen, the amount of fat, the colour and the texture of the meat, and above all, the degree of yake in the meat. The price difference for fresh tuna is about twice that of frozen, comparing USD 28–49 for fresh and USD 14–24 for frozen tuna, though the consumer uses and costs of processing and shipping are also different (Mylonas et al., 2010). The further expansion of bluefin tuna capture-based aquaculture in the Mediterranean Sea from 1997 generated a growing demand of wild specimens that resulted in a major impact on fisheries, a negative impact on the ecosystem and the natural resources and a significant depletion of the stocks. The ICCAT implemented in 1990 a quota system called the Total Allowable Capture (TAC) which has been progressively reduced from 38 000 tonnes in 2004 to 12 900 tonnes in 2012. In 2006, a 15 year recovery plan was adopted by ICCAT. The plan was further revised in 2008 to include multiple levels of monitoring, control and surveillance. The current reduction to a single month between May 15th and June 15th imposed by ICCAT in 2009 resulted in a serious negative impact on fattening operations. The recovery plan has borne fruits, and from 2013 the TAC has been slightly increased up to 13 400 tonnes in 2013 and 2014 and following up to 23 155 tonnes in 2017. Currently, the bluefin tuna fishery is one of the most regulated fisheries in the world. For all ICCAT Contracting Parties, bluefin tuna imports must be accompanied by the Bluefin Tuna Statistical Document (BTSD) and any country re-exporting the tuna must attach the original BTSD along with a re-export document. These documents are used to track the volume of farmed tuna exported to Japan which currently absorbs approximately 90 percent of total farmed tuna.
At the end of the 1990s, researchers from the main European aquaculture research institutions involved in marine aquaculture have been built a group called Domestication of Thunnus thynnus (DOTT) that aimed to domesticate this species. This group obtained funds from the European Union (EU) to organize the First International Symposium of bluefin tuna domestication (DOTT) which was held in Cartagena, Spain. During this conference, most bluefin tuna activities around the world were reported, including: state of the wild populations, bluefin tuna fisheries and landings, and bluefin tuna fattening operations in different parts. In 2002 the DOTT group applied for a new project: REPRODOTT (Reproduction of the bluefin Tuna in Captivity) which aimed to demonstrate the feasibility of the reproduction of bluefin tuna in captivity. The overall objective of this project was to improve the understanding of the reproductive physiology of bluefin tuna as the basis to develop a suitable methodology for the control of its reproduction in captivity, in order to establish a sustainable aquaculture sub-sector. A new project (SELFDOTT: from captured-based to self-sustained aquaculture and domestication of Thunnus thynnus, the Atlantic bluefin tuna) was active between 2008 and 2011. It was funded under the 7th FP (Framework Programme) Cooperation Work Program: Food, Agriculture and Fisheries, and Biotechnology with funding of EUR 3 million, and coordinated by the Spanish Institute of Oceanography (IEO). The main objectives of this project were (i) to substantiate the current knowledge on the reproduction of capture based aquaculture bluefin tuna, (ii) to establish the knowledge base required for controlled development of eggs and larvae and (iii) to establish the knowledge base for the development of suitable and environmentally performing feeds. The SELFDOTT project delivered the proposed objectives, substantiating the results of reproduction of Atlantic bluefin tuna in captivity obtained in the previous projects and establishing baseline knowledge for the production of fingerlings of this species and for the development of more efficient feeds that are more sustainable. Though these projects have had resounding successes, there are still many aspects that must be improved, and, therefore, it is concluded that the large-scale commercial production of this species in a profitable manner is not yet developed sufficiently enough to fuel a new aquaculture industry. In July 2015, the Spanish Institute of Oceanography (IEO) developed an infrastructure for controlling the reproduction of the bluefin tuna. The infrastructure consists of a building of 2 660 m2, consisting of four large tanks: 2 tanks for broodstock (1 tank of 22 m diameter and 9 m depth, 1 tank of 20 m diameter and 9 m depth with a volume of 3 500 and 2 500 m3, respectively), and 2 tanks for juveniles (14 and 8 m diameter, 3 m depth with a volume of 900 and 150 m3); total volume of the 4 tanks is 7 000 m3. This facility, co-funded by the European Regional Development Fund (ERDF), aims to guarantee the consistent availability of bluefin tuna eggs in the quantity and quality needed for the bluefin tuna production for the industry and for research purposes contributing its conservation and supporting and fueling the bluefin tuna research and industrial development. Although the natural populations of bluefin tuna show signs of recuperation in response to the implemented control and recovery program by the ICCAT, it is clear that the limitations on captures will continue in the future. Therefore, any increase in productivity of this species in order to satisfy the demand in quantity and quality as increasingly required by a growing and selective market passes to the production of bluefin tuna via full aquaculture cycle techniques, in the same way as already occurs with species such as seabream, seabass, turbot or salmon.
The capture-based aquaculture of bluefin tuna has contributed to an overexploitation of the natural population and caused the ICCAT to establish a quota system to limit captures in 1999, and significantly revised in 2006. The gradual reduction of these quotas, the increase in the minimum capture size and the established closure system has led to a significant decrease in the fattening sector, in such a way that, despite the fact that clear signs of the recuperation of this species can now be seen, there is general agreement that the future of bluefin tuna aquaculture depends on the closing of the life-cycle in captivity and being able to produce fry in nurseries. In view of the extensive use of low-value fishes as feed, the high feed conversion ratios and related farm management problems (e.g. purchasing, transporting, storage, and distribution of feed fish and corresponding environmental effects), other than the overfishing of the species used as feed, the industry must intensify studies on artificial feed in order to mitigate the problems associated with the use of low-value fishes as feed. In the meantime, however, there is a need to standardize control systems to ensure feed quality and avoid the introduction of potential pathogens. In order to ensure total transparency of the industry and traceability of traded tuna, farmers need to adopt and follow best farming practices throughout the production process.
The development of a specific bluefin tuna code of conduct should be shared by fishers, farmers and importers to ensure the implementation of all management regulations. This could also be a tool for the collection and reporting of bluefin tuna capture-based aquaculture data.
Abascal, F.J., Megina, C. & Medina, A. 2003. Histological and stereological assessment of batch fecundity spawning frequency and maturation of female Atlantic northern bluefin tuna around the Balearic Islands. Cahiers Options Méditerranéennes, 60: 13–14.
Baglin, R. E., and L. R. Rivas. Population fecundity of western and eastern North Atlantic bluefin tuna (Thunnus thynnus). ICCAT Col. Vol. Sci. Pap 6 (1977): 361–365.
Benetti, D., Buentello, A., Partridge, G. (eds). 2015. Advances in tuna aquaculture : from hatchery to market. Academic Press 359 pp.
Brill, R. 1994. A review of temperature and oxygen tolerance studies of tunas pertinent to fisheries oceanography, movements models and stock assessments. Fish. Oceanogr., 3(3): 204–216.
Bushnell, P.G. & Holland, K.N. 1997. Tunas. Virginia Mar. Res. Bull., 29(1&2): 3–6.
Cort, J.L. 1990. Biologia y pesca del atún rojo, Thunnus thynnus (L.), del Mar Cantábrico. Inst. Español Ocean., Publ. Esp., 4: 272 p.
De la Gándara, F., Ortega, A. and Buentello, A. 2016. Tuna Aquaculture in Europe. In: Advances in Tuna Aquaculture. From hatchery to market. Chapter 6. Benetti, D.D. ,Partridge, G.J. & Buentello, A. (Eds.) Elsevier Academic Press, New York.
Dickson, K.A. 1995. Unique adaptations of the metabolic biochemistry of tunas and billfishes for life in the pelagic environment. Env. Biol. Fish., 42: 65–97
FAO. 1983. Species Catalogue. Vol. 2. Scombrids of the world. An annotated and illustrated catalogue of Tunas, Mackerels, Bonitos and related species known to date. Collette, B.B. & C.E. Nauen. FAO Fish. Synop., (125) Vol.2:137 pp.
FAO. 1994. World review of highly migratory species and straddling stocks. FAO Fish. Tech. Pap. 337 : 1-75.
ICCAT. 2008. Recommendation amending the recommendation by ICCAT to establish a multiannual recovery plan for bluefin tuna in the eastern Atlantic and Mediterranean. Madrid, International Committee for the Conservation of Atlantic Tuna, 28 pp.
ICCAT. 2015. Report of the standing committee on research and statistics (SCRS). Madrid (Spain) 28th to 2nd October 2015.
Joseph, J., Klawe, W. & Murphy, P. 1988. Tuna and Billfish – fish without a country. Fourth edition, Inter-American Tropical Tuna Commission (ed), La Jolla, California: 69 pp.
Karakulak, S., Oray, I., Corriero, A., Deflorio, M., Santamaría, N., Desantis, S. and De Metrio, G. 2004a. Evidence of a spawning area for the bluefin tuna (Thunnus thynnus L.) in the Eastern Mediterranean. J. Appl. Ichthyol., 20: 318–320.
Karakulak, S., Oray, I., Corriero, A., Spedicato, D., Suban, D., Santamaria, N. & De Metrio, G. 2004b. First information on the reproductive biology of the bluefin tuna (Thunnus thynnus) in the Eastern Mediterranean. Collective Volume of Scientific Papers ICCAT, 56(3): 1158–1162.
Metian, M., Pouil, S., Boustany, A. and Troell, M. 2014. Farming of Bluefin Tuna-Reconsidering Global Estimates and Sustainability Concerns. Reviews in Fisheries Science & Aquaculture 22(3): 184-192.
Mylonas C.C., De la Gándara F., Corriero A. y Belmonte Rios, A. 2010. Atlantic Bluefin Tuna (Thunnus thynnus) Farming and Fattening in the Mediterranean Sea. Reviews in Fisheries Science, 18(3): 266-280.
Ottolenghi F. 2008. Capture-based aquaculture of bluefin tuna, pp. 169-182. In: Capture-Based Aquaculture, (Lovatelli, A., and P. F. Holthus, Eds.). Rome, Food and Agriculture Organization of the United Nations. 508 pp.
Partridge, G.J. 2013.Closed-cycle hatchery production of tuna. In: Advances in Aquaculture Hatchery Technology, Chapter: 15, Publisher: Woodhead Publishing Limited, Editors: Alan G, Burnell G. Pag. 457-497
Smith-Vaniz W.F. 1986. Scombridae. En: Fishes of the North-eastern Atlantic and the Mediterranean. II. Whitehead P.J.P., Bauchot M.L., Hureau J.C., Nielsen A. y Tortonese E. (Eds.), UNESCO Paris: 981-997.
Webb, P.W. 1984. Body form, locomotion and foraging in aquatic vertebrates. Amer. Zool., 24: 107–120.
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Fisheries and Aquaculture Division
| Fisheries and Aquaculture Division |


 Food and Agriculture Organization of the United Nationsfor a world without hunger
Food and Agriculture Organization of the United Nationsfor a world without hunger



 Towing cage - Fernando de la Gándara-IEO
Towing cage - Fernando de la Gándara-IEO Thunnus thynnus in cage - Fernando de la Gándara-IEO
Thunnus thynnus in cage - Fernando de la Gándara-IEO Fattening - Fernando de la Gándara-IEO
Fattening - Fernando de la Gándara-IEO Harvesting - Fernando de la Gándara-IEO
Harvesting - Fernando de la Gándara-IEO Eggs in embrion - Fernando de la Gándara-IEO
Eggs in embrion - Fernando de la Gándara-IEO Thunnus thynnus junenile - Fernando de la Gándara-IEO
Thunnus thynnus junenile - Fernando de la Gándara-IEO